

Women should be advised to continue contraception until they are post-menopausal or older than 55y old.
If a woman is using a hormonal method of contraception, is amenorrhoeic and wishes to stop between 50-55y old, check their FSH. If FSH is >30 iU/L then they should continue for another 1 year and then stop.
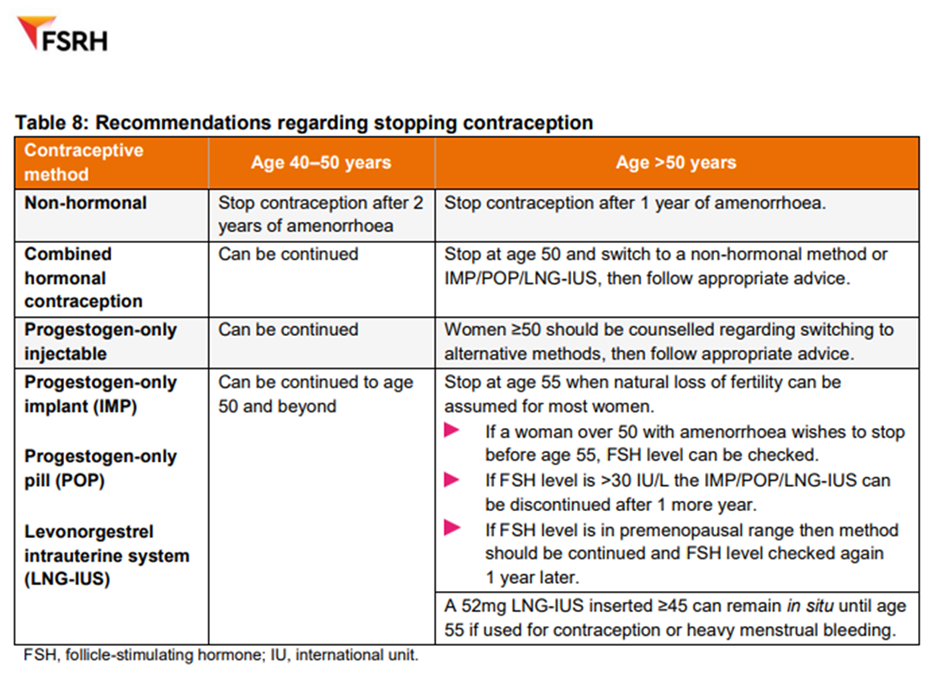
HRT is not contraceptive. Follow FSRH and UK MEC guidance for advice on contraception choices.
Combined hormonal contraception (pills, patch, vaginal ring) may provide menopausal symptom relief for eligible women under the age of 50, instead of HRT.
Generally, progestogen only methods of contraception are not licensed for endometrial protection and additional progestogen is required for this purpose.
|
Type of Contraception + Dose |
Contraceptive main method of Action |
Additional Progestogen required as part of HRT |
|
52mg Levonorgestrel IUD |
Thickens cervical mucus |
No (5 years) |
|
19.5mg, 13.5mg Levonorgestrel IUD |
Thickens cervical mucus |
Yes |
|
Desogestrel PO 75mcg |
Anovulation |
Yes |
|
Norethisterone PO 350mcg |
Thickens cervical mucus |
Yes *off-license 3x tablets (1050mcg) may be used as endometrial protection |
|
Levenorgestral PO 50mcg |
Thickens cervical mucus |
Yes |
|
Drospirenone PO 4mg |
Anovulation |
*No – must omit hormone free interval (HFI) |
|
Medroxyprogesterone Acetate 150mg IM / 104mg SC |
Anovulation |
Yes |
|
Etonogestrel Implant |
Anovulation |
Yes |
*Supported by BMS guidance - https://thebms.org.uk/wp-content/uploads/2024/02/15-BMS-TfC-HRT-preparations-and-equivalent-alternatives-JAN2024-B.pdf
The contraceptive implant has a higher rate of irregular bleeding which would need to be investigated as unscheduled bleeding in women taking HRT. It remains a safe and effective option if this is a patient’s preference.
The progestogen injection is not recommended to be continued after the age of 50 due to cardiovascular and bone effects.
Non-hormonal methods (Cu-IUD, condoms, diaphragm) can be used safely in conjunction with HRT.
Some recommended topical vaginal treatments can damage condoms, this should be discussed as required. Estriol vaginal HRT products and oil-based products generally will affect condom integrity. Estradiol products do not affect condom integrity. Check the product information.
Patient Resources
04-WHC-FACTSHEET-ContraceptionForTheOlderWoman-NOV22-B.pdf (womens-health-concern.org)
References and Resources
UK Medical Eligibility Criteria for Contraceptive Use (UKMEC) | FSRH
Efforts are made to ensure the accuracy and agreement of these guidelines, including any content uploaded, referred to or linked to from the system. However, BNSSG ICB cannot guarantee this. This guidance does not override the individual responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or guardian or carer, in accordance with the mental capacity act, and informed by the summary of product characteristics of any drugs they are considering. Practitioners are required to perform their duties in accordance with the law and their regulators and nothing in this guidance should be interpreted in a way that would be inconsistent with compliance with those duties.
Information provided through Remedy is continually updated so please be aware any printed copies may quickly become out of date.