

Mpox is a viral infection belonging to same family of viruses as the smallpox virus. It is endemic in Central and West Africa and continues to cause outbreaks. There are two distinct groups;
At the time of writing (August 2024) there is a large Clade I Mpox outbreak in central Africa with the threat of imported infections to the UK. When assessing patients with suspected Mpox it is important to identify any risk factors for Clade I/HCID Mpox.
Please see latest updates on Mpox (Monkeypox) on the PHE website:
Mpox - back ground information
The GOV UK webpages hold helpful information that is regularly updated:
Mpox (monkeypox): guidance - GOV.UK (www.gov.uk)
Mpox: case definitions - GOV.UK (www.gov.uk)
Please note that Mpox is a notifiable disease and suspected cases should be reported.
Be alert to the possibility of HCID Mpox (Clade I) in all patients with suspected Mpox if there is a travel history to the Democratic Republic of the Congo (DRC) or other specified countries in the African region. When assessing patients for Mpox, always a take travel history.
See the NHS England advice on patient self-presenting in general practice: NHS England » Clade I mpox (MPXV) pathway actions: patients self-presenting in general practice
The symptoms of Mpox begin 5-21 days (av 6-16 days) after exposure with initial clinical presentation of fever, malaise, lymphadenopathy and headache.
Within 1 to 5 days after the appearance of fever, a rash develops, often beginning on the face or genital area then spreading to other parts of the body. The rash changes and goes through different stages before finally forming a scab which later falls off. An individual is contagious until all the scabs have fallen off and there is intact skin underneath. Treatment for Mpox is mainly supportive. Clade II Mpox is usually a mild clinical illness which self resolves in a few weeks. Clade I Mpox is associated with more severe disease with a documented mortality of 4-11% in a lower income healthcare setting.
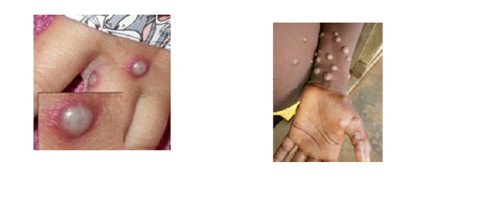
Mpox does not spread easily between people. Spread of Mpox may occur when a person comes into close contact with an animal, human, or materials contaminated with the virus. The virus enters the body through broken skin (even if not visible), the respiratory tract, or the mucous membranes (eyes, nose, or mouth).
Person-to-person spread may occur through direct contact with Mpox skin lesions or scabs; contact with clothing or linens (such as bedding or towels) used by an infected person; or through respiratory transmission, such as coughing or sneezing of an individual with a Mpox rash. Sexual contact was the primary driver of transmission during the 2022 Clade 2 outbreak. This may not be the case with the current Clade 1 outbreak – whilst at the time of writing there has only been one Clade I case reported in Europe, in affected West African countries around two thirds of infected people were under 15 years of age.
Clade I HCID mpox
All patients considered as having possible HCID Mpox of any severity must be discussed with Infectious Diseases team or Infection SpR on call to discuss testing and management.
ID SpR/Consultant 0800-1600 M-F (Bleep 9290 via NBT switchboard) and On call infection SpR outside these times (also via NBT switchboard)
See the following document for further helpful information:
Mpox and 4CMenB vaccine for gonorrhoea (update September 2025)
NHSE has advised that the mpox and 4CMenB for gonorrhoea opportunistic vaccination programmes have been rolled out across sexual health services in the South West.
These vaccines are only commissioned to be delivered by sexual health services. Both are opportunistic and therefore there is no call/recall process for either. Should a patient ask about either of these vaccines, please signpost them to their local sexual health service, who can then assess their eligibility. Practices are not expected to identify eligible patients, nor to promote the vaccines as these are opportunistic.
For further information please contact england.swvast@nhs.net
Patient information on Mpox can be found on the NHS website
NHSE pathway action cards for suspected Mpox. There are four flow diagrams in this document:
Efforts are made to ensure the accuracy and agreement of these guidelines, including any content uploaded, referred to or linked to from the system. However, BNSSG ICB cannot guarantee this. This guidance does not override the individual responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or guardian or carer, in accordance with the mental capacity act, and informed by the summary of product characteristics of any drugs they are considering. Practitioners are required to perform their duties in accordance with the law and their regulators and nothing in this guidance should be interpreted in a way that would be inconsistent with compliance with those duties.
Information provided through Remedy is continually updated so please be aware any printed copies may quickly become out of date.