

Services provided by UHBW at Bristol Royal Infirmary and Weston General Hospital and NBT at Southmead Hospital. The services are for patients who meet the Upper Gastrointestinal Cancer Urgent Suspected Cancer criteria.
Refer Direct to test via ICE for USC (2WW) referrals. Please use this route for suitable patients
Please see link for further NICE Cancer Guidelines 2015 - Upper GI Tract Cancers
At time of referral please issue the relevant patient information leaflet and ensure that appropriate blood tests are arranged, do not delay referral while waiting for blood test results.
Patients may have a diagnostic test as their first appointment ie OGD, CT, USS, MRCP
If advised to refer into MDT, then please submit a USC referral to the relevant secondary care team who will ensure that all the required information is available to enable an effective MDT discussion.
Contact details ADULT USC:
NBT - Tel on 0117 414 0522 / 0536 / 0537 / 0538 or email to cancerservices@nhs.net
UHBW - Tel on 0117 342 7641 / 2 / 3 / 4 or email to Ubh-tr.fast-trackreferrals@nhs.net
Iron Deficiency Anaemia
Please note that iron deficiency anaemia (IDA) alone is not an indication for USC upper GI referral. In these cases it is advised that lower GI investigations should be prioritised. Please see the lower GI 2WW criteria initially as an USC lower GI endoscopy or FIT test may be indicated depending on age and other symptoms. There is also advice on management of IDA in the Anaemia page of Remedy.
If upper GI endoscopy is required then referrals should be sent to a community endoscopy provider (outside of USC pathway).
Please see the Endoscopy page for details.
Oesophago-gastric cancer presents with a number of different symptoms, including dysphagia, often accompanied by pain, acid reflux, loss of appetite and weight loss. An upper abdominal mass may be palpable and the patient may be anaemic.
The first line investigation is upper GI endoscopy which should be accessed as a direct access USC endoscopy referral on ICE unless the patient is unable/unfit to go straight to test.
There is also significant overlap with HPB malignancy so consider whether an USS or CT abdomen is indicated.
NICE USC Referral Criteria
Dysphagia
Patients over 55 with weight loss and at least one of the following;
Upper abdominal mass consistent with stomach cancer
See Upper GI page for indications for non urgent direct access Upper GI endoscopy
LINK here to below information
(NICE [2015] suggests NON-URGENT direct access upper gastrointestinal endoscopy for
or raised platelets with any of;
Nausea, vomiting, Weight Loss, Reflux, Dyspepsia, Upper abdominal pain
Weight loss, Reflux, Dyspepsia, Upper abdominal pain
Iron Deficiency anaemia
Please see guidance on CKS for investigation of IDA, these patients may meet the criteria for the colorectal cancer pathway
UGI surgery clinic for consideration of Gallstone related or reflux related surgical treatment should be made via a routine eRS referral – please see Remedy Website sections for criteria for referral.
Gastroenterology advice or clinic for further assessment of Haematemesis / Iron Deficiency anaemia, treatment‑resistant dyspepsia or reflux, inflammatory bowel disease (IBD) concerns or hepatology conditions (including for deranged LFTs / mild jaundice) and chronic pancreatitis should be made via a routine eRS referral– please see relevant Remedy Website sections for advice and criteria for referral.
Pancreatic cancer can present with a number of different symptoms, and there are often multiple symptoms simultaneously. Symptoms include pain, loss of appetite, and loss of weight. Lesions near the head of the pancreas may lead to obstructive jaundice. Endocrine cancers may produce symptoms from secretion of hormones such as insulin.
Jaundiced Patients over 40y/o
Bilirubin >40 (not deranged LFTS alone)
Request urgent direct access CT scan (to be performed within 2 weeks and refer on Upper GI USC pathway
Bilirubin >100
Refer via an emergency surgical service as these patients need a CT within 24hours which cannot be delivered on an USC pathway
Deranged LFTs +/- Bilirubin <40 is not an USC referral; follow locally agreed pathways for these patients
Patients aged over 60 without jaundice but with weight loss and at least one of the following
Abdominal pain/dyspepsia, Back pain, Diarrhoea or Constipation, Nausea or Vomiting, New‑onset diabetes.
Request urgent direct access CT scan (to be performed within 2weeks and refer on Upper GI USC pathway)
Pancreatic cancer suggested on imaging
Refer USC on ERS, please perform suggested blood tests and attach imaging report if not available on BNSSG ICE.
Please see Pancreatic Cysts for advice on their management.
Suspected Liver or Gallbladder cancer
If identified on imaging refer on Upper GI USC pathway
If patient presents with unexplained possible liver/gallbladder mass request urgent direct access ultrasound (to be performed within 2 weeks) and refer on Upper GI USC pathway.
Gall bladder polyps
The majority of gall bladder polyps do not require fast track referral link to non 2ww page for info
Gall bladder polyps >2cm should be referred on the Upper GI USC pathway
Please request urgent direct access CT scan (to be performed within 2 weeks) for HPB MDT discussion
Non Fast-Track Gall Bladder Polyps:
These patients may be directed to a non-2ww pathway if the findings are not considered concerning.
URGENT /ROUTINE cholecystectomy or imaging follow-up is recommended.
The following should be referred on a URGENT (NON FAST TRACK) pathway to any HPB / UGI surgeon:
Imaging result indicates gall bladder polyp > 1cm or >6mm with any high risk factors:
Age >50
History of PSC
Family history of Gall bladder cancer
Indian Ethnicity
Sessile Polyp >4mm thick
Patients should be referred for ROUTINE cholecystectomy with:
Gallbladder polyp 6 -10 mm and concomitant gallstones in the presence of symptoms
Primary Sclerosing Cholangitis & gallbladder polyp of any size
Advised Imaging follow up for known polyps:
Polyp < 10 mm should have a follow up USS at 6 months.
If not enlarging >2mm or more and still < 10mm then imaging follow up as follows:
< 6mm with above risk factors or 6-9 mm with no risk factors– repeat ultrasound scan every 12 months
<6mm no risk factors – repeat ultrasound scan every 2 years
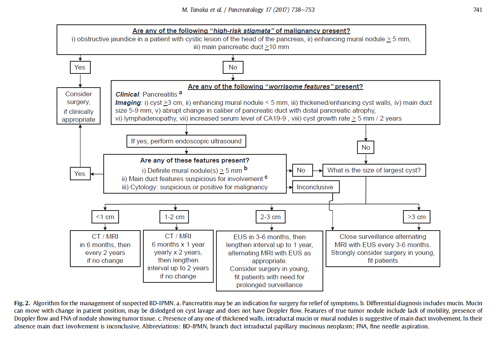
Pancreatic cysts
Refer all pancreatic cysts on the Upper GI USC pathway unless the patient is not well enough for or declines further investigation/treatment.
Please attach imaging report and ensure clinical details are included.
This information will be used to guide whether the pancreatic cyst has a high risk of malignancy. If on review of the imaging the risk is agreed to be low patients will be downgraded to a non cancer pathway and will be informed of this before being followed up in an urgent clinic appointment to discuss interval follow up imaging.
The following criteria will be used to guide the risk of malignancy;
Cyst with high risk factors:
Obstructive Jaundice and cysts in Head of Pancreas
Main pancreatic duct ≥ 10 mm in size
Enhancing mural nodule ≥ 5 mm
Cyst with Worrisome factors:
Cysts > 3cm
Main pancreatic duct ≥ 5 mm in size
Abrupt change in calibre of pancreatic duct with distal pancreatic atrophy
Enhancing mural nodule < 5 mm or thick walled cysts
Cysts with regional Lymphadenopathy
Increased serum level of CA-19-9
Cyst growth rate ≥ 5 mm / 2 years
Please ensure that the following recent blood results are made in parallel with the referral (less than 8 weeks
old):
For all suspected UGI fast track patients: FBC, LFT inc Albumin, U&E inc EGFR
For suspected pancreatic and biliary cancers, include CA 19-9, Clotting
For suspected liver cancer, include Ca19-9, alpha feto protein (AFP), CEA & Clotting
Efforts are made to ensure the accuracy and agreement of these guidelines, including any content uploaded, referred to or linked to from the system. However, BNSSG ICB cannot guarantee this. This guidance does not override the individual responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or guardian or carer, in accordance with the mental capacity act, and informed by the summary of product characteristics of any drugs they are considering. Practitioners are required to perform their duties in accordance with the law and their regulators and nothing in this guidance should be interpreted in a way that would be inconsistent with compliance with those duties.
Information provided through Remedy is continually updated so please be aware any printed copies may quickly become out of date.