Checked: 09-12-2024 by
Vicky Ryan Next Review: 08-12-2026
Breast Cancer Risk - low risk population
Using the MHRA risk table, explain to people around the age of natural menopause that:
- The baseline risk of breast cancer for people around menopausal age varies from one person to another according to the presence of underlying risk factors
- HRT with oestrogen alone is associated with little change in the risk of breast cancer
- HRT with oestrogen and progestogen can be associated with an increase in the risk of breast cancer
- Any increase in the risk of breast cancer is related to treatment duration, with risk increasing from 12 months of use, and reduces after stopping HRT
- HRT does not appear to change breast cancer risk in the first year of use in a baseline population. Between 1-5 years risk is slightly increased, after 5 years use this increase is more significant.
- Micronized Progesterone (MP) and dydrogesterone appear to have a more neutral impact on breast tissue up to 5 years of use and can be offered in line with formulary guidance.
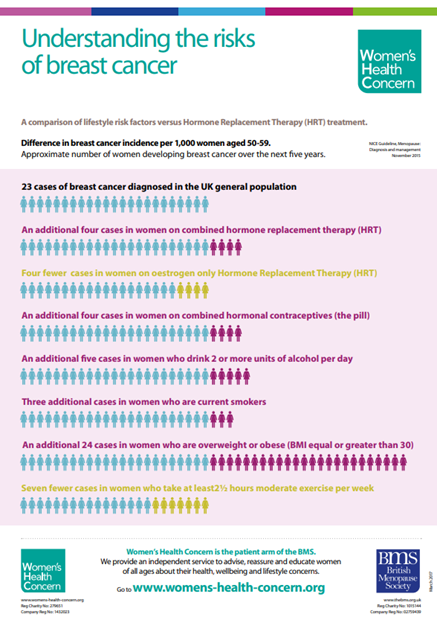
Women’s Health concern also have a helpful infographic - WHC-UnderstandingRisksofBreastCancer-MARCH2017.pdf (womens-health-concern.org)
Moderate / High Risk of Breast Cancer
Offer menopausal women and people with moderate/high risk of breast cancer:
- Information on lifestyle to minimise other risk factors e.g. BMI, alcohol intake, smoking cessation, inactivity
- Take comprehensive family history and follow Remedy guidance regarding need for genetic screening/early mammograms
- If referring to ‘Family Breast Clinic’ or ‘Clinical Genetics’ please await their review prior to starting HRT
- If following an individualised risks vs benefits discussion, a patient would like to start HRT – choose the lowest risk options.
- Micronized Progesterone (MP) and dydrogesterone appear to have a more neutral impact on breast tissue up to 5 years of use and should be offered if women at moderate / high risk of breast cancer are considering HRT use after counselling about non-hormonal vs hormonal options.
Very High Risk of Breast Cancer
- Women with BRCA genes or those with a risk of > 30 % requesting HRT should be referred to a secondary care menopause specialist.
- Do not start HRT
Previous or current breast cancer
Menopausal symptoms with breast cancer or a history of breast cancer
- Stop systemic hormone replacement therapy (HRT) in people who are diagnosed with breast cancer. (Recommend weaning to avoid severe side effects) This includes LNG-IUD’s (e.g. mirena).
- Information and counselling should be offered about the possibility of early menopause and menopausal symptoms associated with breast cancer treatment – this should be discussed by the breast care service.
- Do not use FSH to diagnose menopause whilst using tamoxifen.
- Do not offer HRT to people with menopausal symptoms and a history of breast cancer. If required, these patients should be referred to the complex menopause clinic.
- SSRIs paroxetine, fluoxetine, or sertraline should not be offered to people with breast cancer who are taking tamoxifen.
- Vaginal oestrogens should not be offered to people using aromatase inhibitors.
- Vaginal oestrogens may be considered for women using tamoxifen, or who have stopped adjuvant therapy. Please use advice and guidance platforms within gynaecology if there is uncertainty.
- Women with breast cancer qualify for vaginal moisturisers on prescription in BNSSG.
- Refer patients with a history of breast cancer and poor symptom control or with very poor quality of life to the menopause oncology clinic. Offer alternative interventions (do not start HRT) whilst waiting for the person to be seen.
- Do not recommend soy (isoflavone), red clover, black cohosh, vitamin E or magnetic devices to treat menopausal symptoms in people with breast cancer. These should be avoided in this cohort.
Referral
If indicated, Advice and Guidance and/ or Referrals can be sent to the Complex Menopause Clinic at UHBW. All referrals are triaged and appointments will be booked in the appropriate clinic (complex or oncology). Please see further information regarding referrals to the Menopause Oncology Clinic here: Referrals
Breastfeeding and HRT
There remains no conclusive research on the passage of HRT medication into breastmilk. It appears anecdotally that there is less impact from using transdermal preparations than oral medication. There is a theoretical possibility of reduction in lactation due to the oestrogen contact inhibiting prolactin. Anecdotally HRT has been used by breastfeeding women without impact on the nursling or supply. The decision should be that of the lactating mother after discussion with her healthcare professional.
Vaginal oestrogen is compatible with breast feeding and may be helpful for women experiencing vaginal dryness due to breastfeeding or menopause.
Resources
Patient Resources
01-WHC-FACTSHEET-BreastCancer-NOV2022-C.pdf (womens-health-concern.org)
WHC-UnderstandingRisksofBreastCancer-MARCH2017.pdf (womens-health-concern.org)
Breast Cancer Risk and Hormone Replacement Therapy: What You Need to Know
RESOURCES
www.womens-health-concern.org The patient arm of the British Menopause Society, includes lots of useful factsheets. E.G. breast cancer risk factors and the chart on understanding the lifestyle risk factors for breast cancer during consultations.
12-BMS-TfC-Fast-Facts-HRT-and-Breast-Cancer-Risk-NOV2022-A.pdf (thebms.org.uk)
WHC-UnderstandingRisksofBreastCancer-MARCH2017.pdf (womens-health-concern.org)
08-BMS-ConsensusStatement-Benefits-risks-of-HRT-before-after-a-breast-cancer-diagnosis-MARCH2024-A.pdf (thebms.org.uk)
Overview | Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer | Guidance | NICE
Recommendations | Menopause: diagnosis and management | Guidance | NICE
Efforts are made to ensure the accuracy and agreement of these guidelines, including any content uploaded, referred to or linked to from the system. However, BNSSG ICB cannot guarantee this. This guidance does not override the individual responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or guardian or carer, in accordance with the mental capacity act, and informed by the summary of product characteristics of any drugs they are considering. Practitioners are required to perform their duties in accordance with the law and their regulators and nothing in this guidance should be interpreted in a way that would be inconsistent with compliance with those duties.
Information provided through Remedy is continually updated so please be aware any printed copies may quickly become out of date.


