

Please see this quick reference guide for commonly sent samples from primary care. This guide applies to all infection samples sent from primary care in BNSSG. The samples are all processed at NBT.
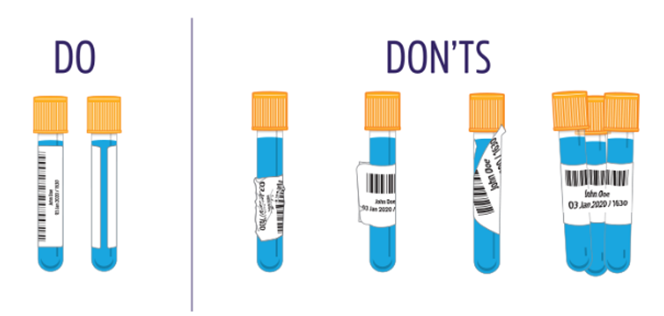
As part of Infection sciences commitment to improve patient care and environmental sustainability we are reducing rejected specimens. Many rejected specimens are due to incorrect specimen container. This could result in a delay in diagnosis and treatment for patients and leads to unnecessary waste and transport costs.
Please ensure all samples
To request testing kits and tubes please contact pathologyconsumablessouthmead@nbt.nhs.uk
For any other queries or suggestions for improvements relating to the below please email microbiology@nbt.nhs.uk
For a full list of Pathology testing repertoire including turnaround times please see
Test Information | North Bristol NHS Trust (nbt.nhs.uk)



To request testing kits and tubes please contact: pathologyconsumablessouthmead@nbt.nhs.uk
For any other queries or suggestions for improvements relating to the below please email: microbiology@nbt.nhs.uk
For a full list of Pathology testing repertoire including turnaround times please see
Test Information | North Bristol NHS Trust (nbt.nhs.uk)
Efforts are made to ensure the accuracy and agreement of these guidelines, including any content uploaded, referred to or linked to from the system. However, BNSSG ICB cannot guarantee this. This guidance does not override the individual responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or guardian or carer, in accordance with the mental capacity act, and informed by the summary of product characteristics of any drugs they are considering. Practitioners are required to perform their duties in accordance with the law and their regulators and nothing in this guidance should be interpreted in a way that would be inconsistent with compliance with those duties.
Information provided through Remedy is continually updated so please be aware any printed copies may quickly become out of date.