Checked: 10-05-2024 by
Vicky Ryan Next Review: 10-05-2026
Overview
Elevated haemoglobin / haematocrit has a wide differential diagnosis:
- Secondary causes (such as hypoxic lung disease, cyanotic heart disease, smoking, obstructive sleep apnoea and erythropoietin-secreting tumours). Venesection not usually indicated.
- Relative polycythaemia resulting from plasma depletion (e.g. diuretics, alcohol); lifestyle modification required.
- Polycythaemia vera (primary polycythaemia).
The threshold for therapeutic intervention with venesection or cytoreductive therapy in an individual patient depends on the cause, associated symptoms and thrombotic risk factors. For most secondary or relative causes of polycythaemia there is no evidence to support intervention with venesection. Co-existing iron deficiency can sometimes mask the presence of polycythaemia vera.
The JAK2 V617F mutation is detectable in over 95% of patients with PV. Where this mutation is absent, it is a reliable indicator that the patient does not have PV and that secondary causes are implicated.
Before referral
Indications for testing
- Haematocrit* > 0.52 in males
- Haematocrit* > 0.48 in females
*Testing on at least two separate occasions > 3 months apart. Ensure no/minimal tourniquet venostasis for blood samples.
Assessment and Investigations in primary care prior to referral
Assessment:
- More than one similar result? Assessed on sample taken with no / minimal tourniquet time.
- Any history of chronic lung disease, obstructive sleep apnoea, congenital cardiac disease?
- Any history of arterial or venous thrombosis?
- Other arterial risk factors? Hypertension?
- Smoking, alcohol and medications (especially diuretics)
- Any generalised pruritus or splenomegaly?
- Is the white cell count and/or platelet count elevated
Investigations in primary care:
- SaO2, blood pressure and urine dipstick
- Repeat FBC uncuffed, renal and liver function, glucose, diabetes screen
- Consider CXR or other respiratory investigations according to symptoms
- Erythropoietin level (“erythropoietin” on ICE, yellow top clotted sample)
- JAK2 mutation analysis (optional) - EDTA sample and request as follows: UHBW ICE - search “JAK2” to find tick box . NBT ICE- Under GP Path - Haematology section - JAK2 mutation - download and complete referral form. Bristol Haemato-Oncology Diagnostic Request Form | North Bristol NHS Trust (nbt.nhs.uk) this page has the request form on. Send 1x EDTA with the form in the bag. On the form tick "genetic testing" and on the back under MPN M85 tick JAK2 box. The results will take around 2 weeks.
Who to refer
Criteria for referral to secondary care:
- All JAK2 V617F positive cases
- JAK2 V617F negative cases, and
- Unprovoked recent arterial or venous thrombosis (including DVT / PE, CVA / TIA, MI / unstable angina, PVD) or neurological symptoms such as loss of vision. For urgent referral without waiting for JAK2 result
- High/low erythropoietin level AND no secondary cause
- Elevated platelet count and/or white cell count
- Confirmed extreme raised haematocrit (Male >0.600, Female >0.560) in the absence of congenital cyanotic heart disease or hypoxic lung disease. For urgent referral without waiting for JAK2 result
- Consider A&G input for patients with elevated haematocrit who are JAK2 V617F negative in association with: past history of arterial or venous thrombosis, splenomegaly, pruritus, elevated white cell or platelet counts.
Management in primary care:
- Appropriate for patients who are :
- JAK2 V617F negative, with
- Normal erythropoietin level
- Or clear secondary cause present irrespective of erythropoietin level
- Management of secondary polycythaemia in primary care:
- Low dose aspirin not indicated
- 6-12 monthly FBC monitoring
- Modify known associated lifestyle factors: smoking, alcohol
- Use non-diuretic anti-hypertensive agents, reduce/stop anabolic steroid use
- Respiratory review if respiratory disease to optimise medical care
- Cardiology review if cyanotic congenital heart disease
Discharge policy
- Following completion of investigation, only those cases requiring venesection or cytoreductive therapy will remain under outpatient follow-up.
- All other cases will be discharged with a suggested frequency of FBC monitoring and a clearly-stated threshold haematocrit for re-referral.
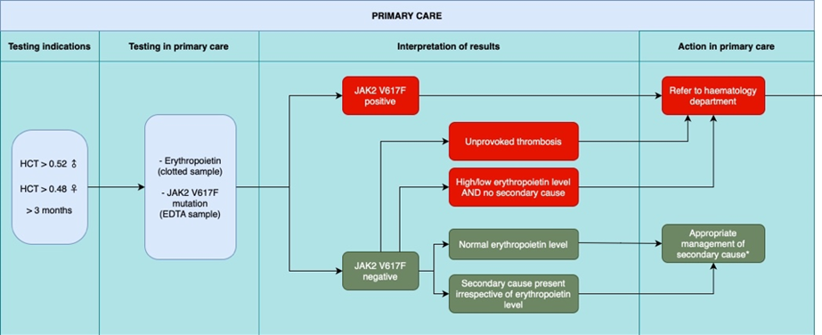
Investigation and management of adult patients presenting with polycythaemia in primary care. From SW MPN Group guideline 2023 Dr Mahdi et al.
Red Flags
Raised haematocrit with:
- Recent arterial or venous thrombosis (incl. DVT/PE, CVA/TIA, MI/unstable angina, PVD)
- Neurological symptoms (e.g. loss of vision)
Referral
URGENT ADVICE: 9am to 5pm via hospital switchboard for haematology SpR. ONLY for emergency advice. Out of hours and weekends – emergency advice may be obtained from the on-call haematology clinician via hospital switchboard.
NON-URGENT ADVICE: use Haematology Advice and Guidance service via e-RS. Your query should be responded by a consultant haematologist within 3 working days.
REFERRAL: via e-RS or Haematology - USC (2WW) as indicated.
Minimal information: the referral letter should include abnormal clinical findings (location, size, any associated features) and any abnormal full blood count results or other relevant test results, particularly if these investigations were not done in laboratories of the hospital to which the referral is made.
Other haematology referrals: most new referrals will go to one of the general haematology clinics but may be triaged to a specialist clinic.
Resources
BSH guideline - Diagnosis and management of polycythaemia vera 2018: British Journal of Haematology | Wiley Online Library
Efforts are made to ensure the accuracy and agreement of these guidelines, including any content uploaded, referred to or linked to from the system. However, BNSSG ICB cannot guarantee this. This guidance does not override the individual responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or guardian or carer, in accordance with the mental capacity act, and informed by the summary of product characteristics of any drugs they are considering. Practitioners are required to perform their duties in accordance with the law and their regulators and nothing in this guidance should be interpreted in a way that would be inconsistent with compliance with those duties.
Information provided through Remedy is continually updated so please be aware any printed copies may quickly become out of date.


