

NICE technology appraisal (1) in December 2024 advised that Tirzepatide (Mounjaro) should be available in primary care for weight management and NHS England have been working towards implementation provisionally from 23rd June 2025.
The BNSSG Weight Management Group (including representatives from BNSSG ICB, GPCB and Avon LMC) have been developing the delivery model and infrastructure so that tirzepatide can be delivered locally in primary care. The following information is to support Practices to prescribe tirzepatide in weight management.
A delivery model and offer of a LES to practices has been shared with BNSSG Practices. The delivery model is shown below.
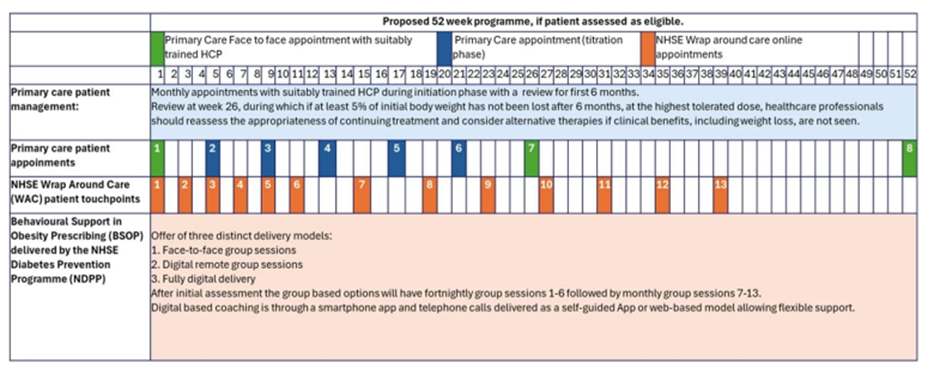
WAC is mandated by NICE alongside tirzepetide prescribing (1) and should encompass nutrition and dietetic advice, physical activity and functional movement, behavioural support and lifestyle change tools.
NHSE have commissioned an interim WAC provider to allow systems to mobilise a weight management service in primary care through the NHSE Diabetes Prevention Programme (NDPP); in BNSSG this is Living Well Taking Control.
Patients will be provided with Behavioural Support for Obesity Prescribing (BSOP) accessed from primary care as a 9-month programme. Patients will be offered a choice of three delivery models:
Please complete the attached referral form (also available on EMIS).
**Please note: Tirzepatide should not be prescribed for weight management if the patient does not agree to engage with WAC.**
An overview of the patients journey once referred to the programme can be found here
The primary care eligibility criteria for the NHSE cohort 1 in Year 1 are as follows*:
Use a lower BMI threshold (usually reduced by 2.5 kg/m2) for people from South Asian, Chinese, other Asian, Middle Eastern, Black African or African-Caribbean ethnic backgrounds.
* NICE technology appraisal TA1026 recommends tirzepetide BMI ≥ 35 and 1 or more weight related comorbidities but the recommended implementation from NHSE for primary care at this stage is as above. Patients meeting the NICE TA criteria can be considered for referral to Tier 3 & 4 Weight Management Service - BNSSG , if appropriate.
We recommend that the Ardens ‘NHS Obesity Medication Pathway’ template is used as a clinical tool to support assessment of eligibility, medication initiation, medication review and record of wraparound pathway. This template also supports the key metrics that NHSE require to be completed to support funding for the ICB. See Tirzepatide BNSSG Prescribing Guidance for further information and guide to completion.
A live Teams webinar for Practices was run on Wednesday 11 June. This has been recorded and is available below in addition to FAQs from the webinar.
Training and Prescribing
NHSE advice for patients who have accessed tirzepatide privately and are requesting an NHS prescription (NHSE primary care bulletin 14.8.25)
'For people that have previously accessed tirzepatide through a private provider, the NHS may only continue treatment if, following an assessment by the Integrated Care Board’s commissioned weight management service, the individual meets the eligibility criteria at the time they present to the NHS.
If a person seeking treatment does not meet the eligibility criteria in the NHS, they should be advised of this. We recognise this may be disappointing for people. NHS teams, including primary care providers, could provide reassurance to the person that stopping tirzepatide, that is being taken for its licensed weight loss indication, is not known to cause withdrawal symptoms, but that they should continue, where appropriate, a reduced-calorie diet and increased physical activity if they want to reduce the risk of weight regain.
Any person presenting to an NHS service with questions about their private tirzepatide prescription, including stopping or tapering off the drug, should be directed to speak with their private provider.'
NHS Behavioural Support for Obesity Prescribing - Prescriber Leaflet
BNSSG ICB information
New medication for weight loss: Tirzepatide (Mounjaro) BNSSG ICB website
There is also information on the NHSE website:
NHS Behavioural Support for Obesity Prescribing - Patient Leasflet
Efforts are made to ensure the accuracy and agreement of these guidelines, including any content uploaded, referred to or linked to from the system. However, BNSSG ICB cannot guarantee this. This guidance does not override the individual responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or guardian or carer, in accordance with the mental capacity act, and informed by the summary of product characteristics of any drugs they are considering. Practitioners are required to perform their duties in accordance with the law and their regulators and nothing in this guidance should be interpreted in a way that would be inconsistent with compliance with those duties.
Information provided through Remedy is continually updated so please be aware any printed copies may quickly become out of date.